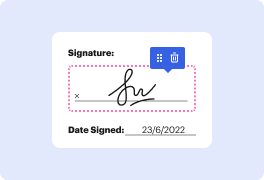

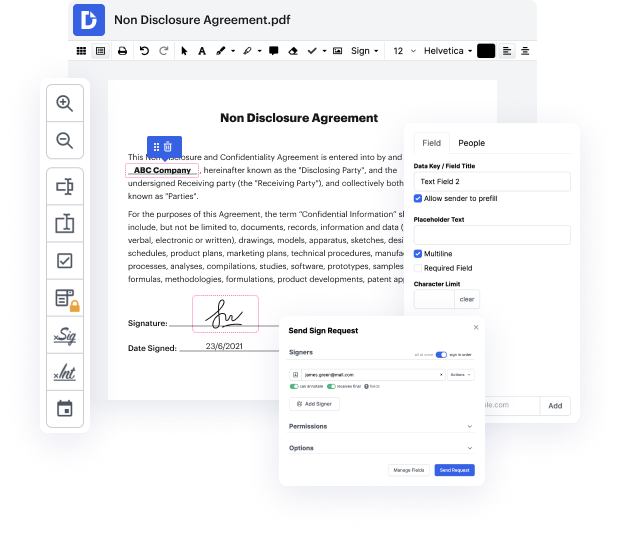
Time is a crucial resource that each organization treasures and attempts to convert in a benefit. In choosing document management application, focus on a clutterless and user-friendly interface that empowers consumers. DocHub delivers cutting-edge instruments to maximize your document administration and transforms your PDF file editing into a matter of one click. Remove Initials Field in the Clinical Trial Agreement with DocHub to save a lot of time as well as enhance your productiveness.
Make PDF file editing an easy and intuitive operation that helps save you plenty of precious time. Effortlessly alter your documents and give them for signing without the need of looking at third-party software. Focus on relevant tasks and increase your document administration with DocHub right now.
hello everyone and welcome to the second ctis bite-sized talk on the initial clinical trial application my name is sarah scales im a change management officer here at ema and i want to first introduce the concept of the bite size talks today then ill explain a little a few housekeeping rules for the session before introducing the ctis experts who will be presenting today so on the concept of the byte size talks each bite-sized talk covers a specific ctis functionality and as seen on the agenda slide here the ctis experts will present the functionality and demonstrate it in the ctis application throughout the session there will be an opportunity to ask questions and if we go to the next slide youll see a few housekeeping rules so first of all we will be using slido as we typically do to collect questions for this event here on this slide you will see how to use slido you can go to w and use the event code march24 you can also use the qr code that youre seeing on your screen and as