
Working with papers means making small corrections to them every day. At times, the job goes nearly automatically, especially when it is part of your day-to-day routine. However, in some cases, working with an unusual document like a Professional Medical Consent may take valuable working time just to carry out the research. To ensure every operation with your papers is trouble-free and quick, you need to find an optimal modifying tool for such tasks.
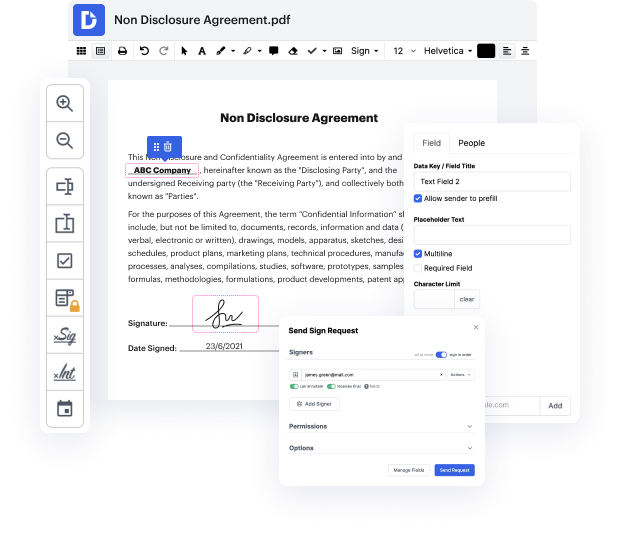
With DocHub, you are able to learn how it works without taking time to figure it all out. Your tools are organized before your eyes and are easy to access. This online tool does not require any sort of background - education or experience - from its customers. It is ready for work even when you are new to software traditionally used to produce Professional Medical Consent. Easily create, edit, and send out papers, whether you work with them every day or are opening a new document type for the first time. It takes minutes to find a way to work with Professional Medical Consent.
With DocHub, there is no need to research different document kinds to learn how to edit them. Have the go-to tools for modifying papers close at hand to improve your document management.
Before participating in the study, it is essential to understand it fully. Researchers will provide an informed consent document that includes details such as study length, number of visits, medications, and procedures. This document also outlines expected outcomes, benefits, risks, and other important information. If needed, a translator can be provided. Researchers will review the document with you, answer your questions, and allow you to decide on participation after talking with your family. Signing the informed consent indicates voluntary agreement to take part in the study, with the option to leave at any time for any reason.