
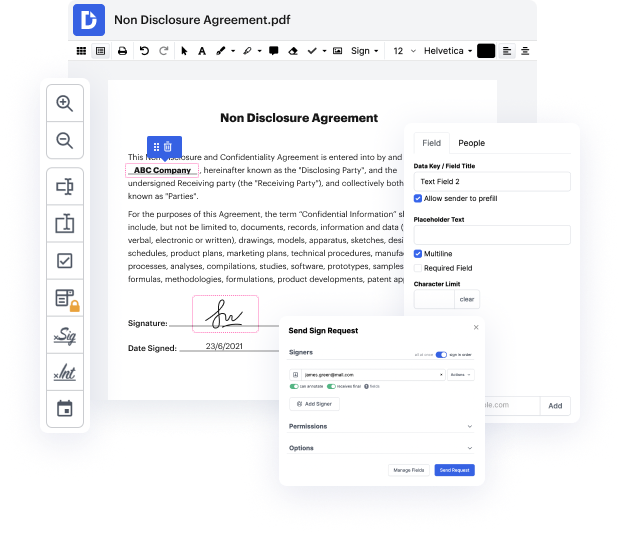
Are you looking for an easy way to join image in Clinical Trial Agreement Template? DocHub provides the best platform for streamlining form editing, certifying and distribution and form execution. Using this all-in-one online platform, you don't need to download and install third-party software or use complex file conversions. Simply add your form to DocHub and start editing it quickly.
DocHub's drag and drop user interface allows you to quickly and quickly make changes, from simple edits like adding text, photos, or graphics to rewriting entire form parts. You can also sign, annotate, and redact paperwork in just a few steps. The solution also allows you to store your Clinical Trial Agreement Template for later use or convert it into an editable template.
DocHub provides more than just a PDF editing system. It’s an all-encompassing platform for digital form management. You can utilize it for all your paperwork and keep them secure and easily accessible within the cloud.
Hello. My name is Stacey Arnold and I am a Results Team Subject Matter Expert for ClinicalTrials.gov. ClinicalTrials.gov is a part of the National Center for Biotechnology Information in the National Library of Medicine at the National Institutes of Health. Today Ill be discussing Registering and Reporting Results to ClinicalTrials.gov. During this presentation, Ill cover the following topics: first, Ill provide a rationale for registering clinical trials and reporting summary level results to ClinicalTrials.gov; next, Ill give an overview of registration and results reporting; then, Ill review an online training platform that has been developed to facilitate the registration and reporting process, the PRS Guided Tutorials; and finally, Ill describe an effort that is underway to modernize the ClinicalTrials.gov website and PRS database. Why register clinical trials and report summary level results? One of the most compelling reasons for registering clinical trials is the Internat