
Not all formats, including QUOX, are created to be effortlessly edited. Even though many features will let us edit all document formats, no one has yet created an actual all-size-fits-all solution.
DocHub offers a straightforward and streamlined solution for editing, taking care of, and storing paperwork in the most popular formats. You don't have to be a tech-savvy user to fill in initials in QUOX or make other tweaks. DocHub is robust enough to make the process easy for everyone.
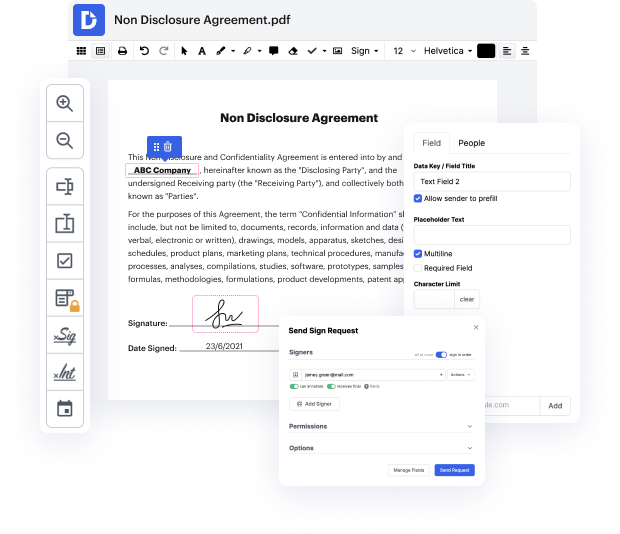
Our feature allows you to alter and tweak paperwork, send data back and forth, generate dynamic forms for information collection, encrypt and shield documents, and set up eSignature workflows. Additionally, you can also create templates from paperwork you utilize frequently.
You’ll locate plenty of additional tools inside DocHub, such as integrations that let you link your QUOX document to a variety business applications.
DocHub is a simple, fairly priced way to manage paperwork and simplify workflows. It offers a wide range of capabilities, from generation to editing, eSignature professional services, and web document creating. The application can export your files in many formats while maintaining maximum security and following the maximum information protection requirements.
Give DocHub a go and see just how easy your editing process can be.
letamp;#39;s look at a chemical reaction and will cast the change and concentration over time the rate of the reaction of a reactant in terms of the change in concentration over time for a product now the change in concentration over time is the slope of the concentration versus time curve and we could start at any initial concentrations and let this reaction go in this case how does the initial rate of change of H+ concentration relate to the zinc ion concentration think about that for a minute and make a selection letamp;#39;s look at a possible explanation for each answer a the rate should be the opposite sign for the other reactant or be the rate should be the same they donamp;#39;t depend on snow kiama tree or see initial rates depend upon snow kiama tree and h plus is consumed it twice the rate think about those three and make a selection weamp;#39;re talking about writing an initial rate for a chemical reaction and we can write initial rates in terms of changes in concentrat