
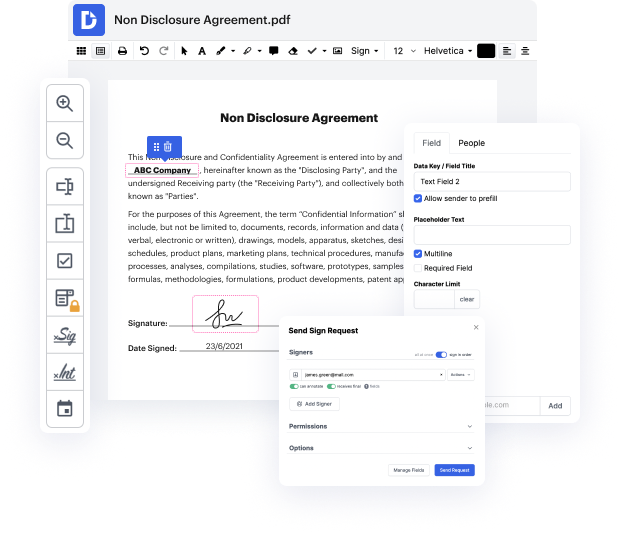
Browsing for a specialized tool that deals with particular formats can be time-consuming. Despite the huge number of online editors available, not all of them are suitable for Xht format, and certainly not all allow you to make adjustments to your files. To make matters worse, not all of them give you the security you need to protect your devices and documentation. DocHub is an excellent answer to these challenges.
DocHub is a well-known online solution that covers all of your document editing requirements and safeguards your work with bank-level data protection. It works with different formats, such as Xht, and allows you to edit such paperwork quickly and easily with a rich and user-friendly interface. Our tool meets important security certifications, such as GDPR, CCPA, PCI DSS, and Google Security Assessment, and keeps enhancing its compliance to provide the best user experience. With everything it provides, DocHub is the most trustworthy way to Faint initials in Xht file and manage all of your individual and business documentation, irrespective of how sensitive it is.
Once you complete all of your modifications, you can set a password on your edited Xht to make sure that only authorized recipients can open it. You can also save your paperwork containing a detailed Audit Trail to check who made what edits and at what time. Choose DocHub for any documentation that you need to edit safely. Sign up now!
hi Im professor Clements and today the problem were gonna be working on is titration of a weak acid with a strong base and for this problem Ive chose formic acid just randomly essentially and were gonna titrate 25 milliliters of 0.2 molar formic acid with 0.4 molar sodium hydroxide and it turns out there are several points to a titration that were going to be worried about so this video is going to come in several parts one of the things we might be concerned about is whats the initial pH when we put the formic acid on our bench top what is going to be the initial pH of that and it turns out that problem is relatively simple if you remember how to do a weak acid because formic acid is simply a weak acid and so even though its a titration problem this part of its fairly simple and were just gonna look at how do we solve for the pH of a weak acid so we take our formic acid we go ahead and we look up in a table that the KA for formic acid is 1.8 times 10 to the minus fourth and y