
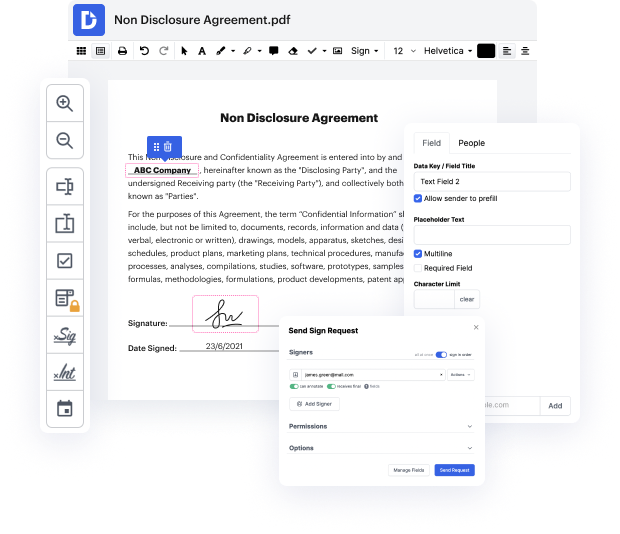
Getting full control of your files at any moment is essential to ease your day-to-day tasks and improve your productivity. Accomplish any objective with DocHub tools for document management and convenient PDF editing. Access, change and save and incorporate your workflows with other safe cloud storage services.
DocHub provides you with lossless editing, the chance to work with any formatting, and safely eSign documents without the need of looking for a third-party eSignature alternative. Make the most from the document management solutions in one place. Consider all DocHub functions today with your free account.
The fall clinical trials toolkit series kicks off with a focus on clinical trial agreement review and negotiation. Rachel Humberson, Interim Assistant Director from the Office of Sponsored Programs, and Julie, a negotiator with the same office, will lead the discussion. Continuing education credits (CEs) are available for WVU employees; those interested should provide their name and email in the chat for attendance purposes. Participants are encouraged to submit questions via chat, which will be addressed during the presentation.