
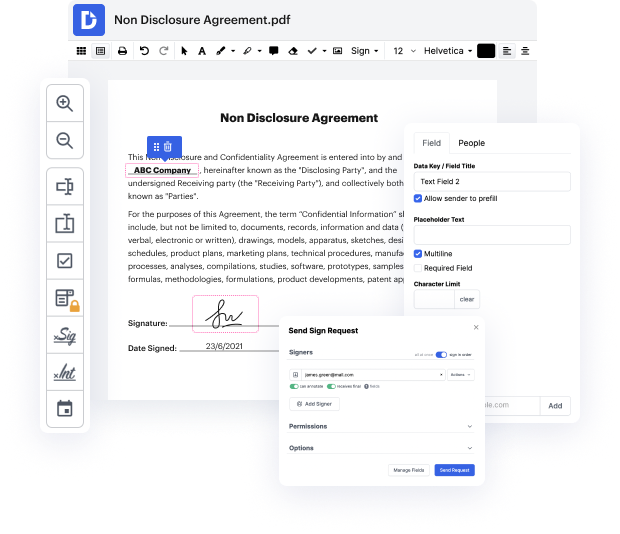
People often need to cut question in xml when managing forms. Unfortunately, few programs offer the options you need to accomplish this task. To do something like this normally requires alternating between a couple of software applications, which take time and effort. Luckily, there is a platform that suits almost any job: DocHub.
DocHub is a perfectly-built PDF editor with a full set of useful functions in one place. Modifying, signing, and sharing forms becomes easy with our online solution, which you can access from any internet-connected device.
By following these five easy steps, you'll have your adjusted xml quickly. The user-friendly interface makes the process quick and efficient - stopping jumping between windows. Start using DocHub now!
hello everyone welcome back to this video series on clinical SAS programming in this video we will see how define.xml would look like for a sdtm submission so for every clinical trial so we collect data using the case report forms and that data we generally call it as draw data and then so we transform our organize the data collected or data present in raw data sets into sdtm structure and programmers use the programming specification to transform the raw data into sdtm datasets and these sdtm data sets are converted into xpt files and then submitted to the regulatory authorities along with the xpt files which contain the actual sdtm data so we also need to submit uh defined.xml annotated case report form and also the study data reviews guide so what does define.xml contain so in order to transform raw data into a regime data sets we have a document called specification which actually contains the information which will be used to uh in which will be used to transform raw data into hdt