
Not all formats, including HWP, are created to be quickly edited. Even though many tools will let us edit all file formats, no one has yet created an actual all-size-fits-all tool.
DocHub offers a straightforward and streamlined tool for editing, taking care of, and storing papers in the most popular formats. You don't have to be a technology-savvy user to conceal substance in HWP or make other tweaks. DocHub is robust enough to make the process simple for everyone.
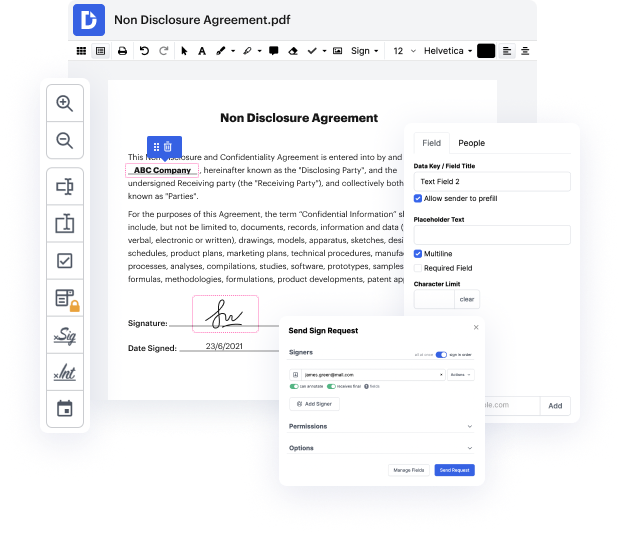
Our feature enables you to alter and edit papers, send data back and forth, generate interactive documents for data gathering, encrypt and safeguard paperwork, and set up eSignature workflows. Additionally, you can also generate templates from papers you utilize on a regular basis.
You’ll locate a great deal of other functionality inside DocHub, such as integrations that allow you to link your HWP file to a wide array of business apps.
DocHub is a straightforward, fairly priced way to manage papers and improve workflows. It offers a wide selection of features, from generation to editing, eSignature professional services, and web form developing. The program can export your paperwork in multiple formats while maintaining highest security and adhering to the highest data security standards.
Give DocHub a go and see just how simple your editing transaction can be.
i thank you for that kind introduction for the next topic weamp;#39;ll be discussing drug substance facilities in particular hidden facilities and critical intermediate facilities used in the manufacturing of drug substances again my name is cassandra avalard iamp;#39;m a consumer safety officer from the office of pharmaceutical manufacturing assessment my colleague way from the office of new drug products weamp;#39;ll give the second half of this presentation which focuses on critical intermediates as a reminder please type any questions in the q a box so we can answer these during the live q a following the presentations so when it comes to drug substance facilities often there is some confusion or lack of clarity on which facilities involved in manufacturing need to be listed in applications during this presentation weamp;#39;ll attempt to clarify which facility should be listed the following facilities in the dmf uh are recommended to be included on the nda and or a nda 356h fo